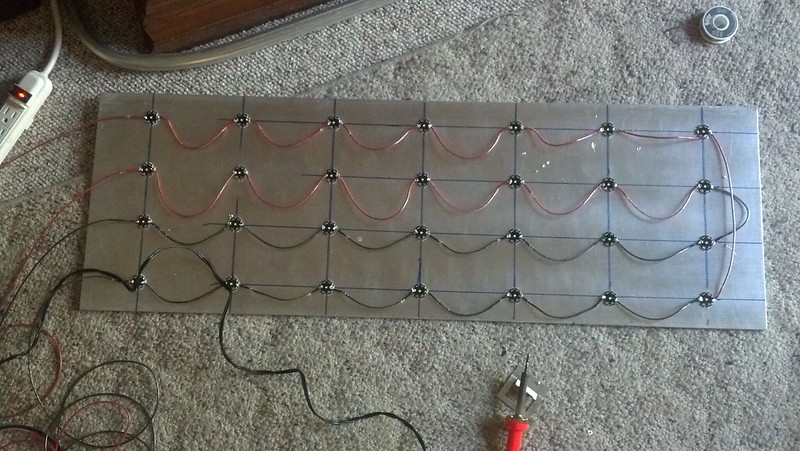
Sorry for the megabump/necro, but I thought it would be appropriate to update/conclude the thread for completeness sake:
After having a lot of trouble with the 2 earlier versions, I ended up with this:
I made a stand out of conduit piping with a bender. I tried using zip tie mounts and wire to mount them, but they kept coming off, so I decided just to use clamps. There's 2 120mm fans on the top running off a ac/dc adapter. I have another ac/dc adapter going to the controller, and the final plug splits to go to the meanwells. The fans/polycarb plate are more or less balancing on the four mounting wires. I believe that the light is sitting high enough off the tank to forego a splash guard this time around, but I did hot glue all soldered joints. I added bullet connectors and quick links late in the design so I could remove the light for maintenance/repairs, but I might replace the quick links with a carabiners. Once I mount another 120mm fan onto the electronics box, I'll be finished.
Here is the final parts list
I'm using 28x (2x strings of 14)5W CREE XP-G R5 5W Cool White LED
CREE XP-G R5 5W Cool White LED - Rapid LED
2x Mean Well ELN-60-48P dimmable driver
Mean Well ELN-60-48P dimmable driver - Rapid LED
1x DDC-01 PWM Controller
DDC-01 PWM Controller - Rapid LED
1x 18x36x.25" 6061 aluminum plate
~30 ft conduit piping
1 desk fan for the meanwells (they get sort of hot).
28x bergquist thermal pads
Berquist Thermal Pad (set of 10) - Rapid LED
bunch of zipties
2x ziptie mount
Amazon.com: Startech HC102 Self Adhesive Cable Tie Mounts (Pack of 100): Electronics
1x 18x24x.125" polycarbonate plate
2x antec 120mm fans
 looks good.
looks good.looks good.