You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
PAR
- Thread starter Mifsud
- Start date
The friendliest place on the web for anyone with an interest in aquariums or fish keeping!
If you have answers, please help by responding to the unanswered posts.
If you have answers, please help by responding to the unanswered posts.
aqua_chem
Aquarium Advice Addict
Spectrometers are better for more qualitative work, or quantitative if calibrated correctly. PAR is measured with photometers rather than spectrometers, although photons are only measured within a certain range based on the absorptive capabilities of plants (400-700 nm). You'll need to measure the photon flux within that spectral range, termed photosynthetic photon flux.
Mifsud
Aquarium Advice Freak
I have a calibrated spectrometer so I can count selectively photons in the range between 400 and 700nm, but does each photon carry the same weight even though green photons are less useful for photosynthesis than blue?
aqua_chem
Aquarium Advice Addict
You're describing PUR rather than PAR. PAR measures all photons between 350-750 (I think I messed up the numbers last time), while PUR measures photons specifically in the portions of the spectrum that plant dyes can absorb photons, as such:

However, PUR is more complicated to measure for a number of reasons:
A) It requires more sensitive equipment, eg the photospectromer such as you have vs a simple photometer that can be had for ~$100
B) It varies based on the pigment composition of plants
C) We use PAR, all our numbers are in PAR, and we cannot interconvert the two at all.

However, PUR is more complicated to measure for a number of reasons:
A) It requires more sensitive equipment, eg the photospectromer such as you have vs a simple photometer that can be had for ~$100
B) It varies based on the pigment composition of plants
C) We use PAR, all our numbers are in PAR, and we cannot interconvert the two at all.
aqua_chem
Aquarium Advice Addict
Additionally, how the heck are you calibrating a spectrometer to measure a number of photons?
Mifsud
Aquarium Advice Freak
I used a standard to calibrate the spectrometer for irradiance. If I measure the irradiance at a given wavelength, then I can calculate the number of photons with Planck's constant. It's elementary Watson.
It sounds to me like if I count all of the photons between 350 and 750 nanometers, then I will have measured the PAR. Does that sound correct?
It sounds to me like if I count all of the photons between 350 and 750 nanometers, then I will have measured the PAR. Does that sound correct?
Sk3lly
Aquarium Advice Addict
Wow this is a very specialised thread.
Subscribed for the interesting read and to learn
Subscribed for the interesting read and to learn
Mifsud
Aquarium Advice Freak
I created this thread because I am designing LED fixtures for my aquarium. I've a planted tank and am not ready to shell out the big bucks for an LED fixture, but more so because I enjoy this type of thing.
My objective is to build a fixture comparable to the $100 Finnex Ray 2 (24" Model) for half of the material price. (My labor will be free)
My first stop on this journey was to select and test lamps. I found these Chinese waterproof fog lights on ebay. Advertised for vehicle use, I figured they'd be very bright. I got 4 lamp sets delivered to my home for $15.

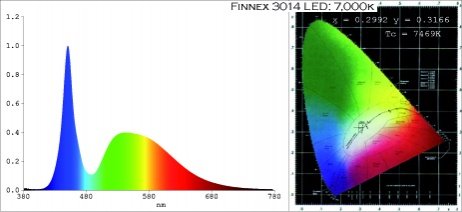
I know that all lamps are not equal, some enhance plant growth more than others. With some experience in a lab, I wanted to test these lamps to characterize their ability to support photosynthesis. I found this thread on another forum with seemingly reliable info on the Finnex Ray 2. Finnex RAY II & FugeRAY PAR Data
Finnex's LED Spectrum from the thread (And website?)
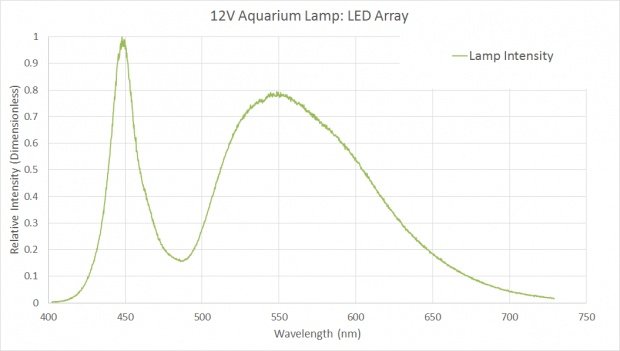
Measured Spectrum of Chinese Fog Lamp LED Spectrum
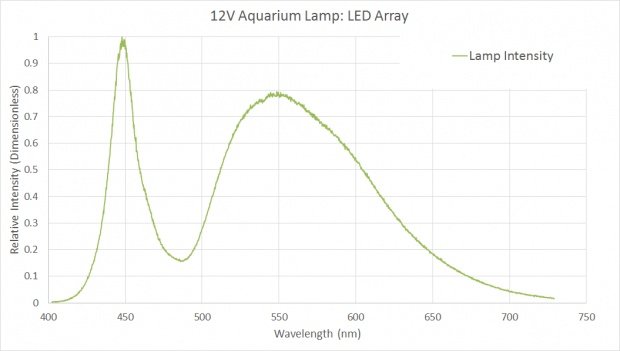
So far so good. It looks like I am on the right path. But now I've got to measure the PAR of these Chinese fog lamps for comparison with Finnex's. That's when I opened this thread.
In the lab, I don't have access to a PAR sensitive photometer, and the premise of this thread is I am too cheap to go out and buy one. But I was able to get my hands on a calibrated spectrometer. (This is a VERY expensive device, but I found myself able to borrow one.) My strategy was to use the spectrometer, a few assumptions, and some undergraduate knowledge of physics to crank out a PAR measurement.
First, the assumption:
I assumed that 100% of the power that entered the lamp was converted into light. This isn't true, but truth is not too far off. I measured each lamp dissipated 3.5W, so I assumed that the total amount of light power produced equaled 3.5W.
Then, the spectrometer:
I measured the spectrum of light produced by my new Chinese fog lamps, you can see the graph produced above. The area bound by this curve I assumed to be equal to the 3.5W I measured earlier.
Finally, the physics:
In the early 20th century a few scientists determined that the wavelength a photon was related to the energy of that photon. Having measured the energy delivered by a number of photons with my spectrometer, I divided by the energy per photon to find the number of photons.
Adding all of the photons across all wavelengths of light between 400 and 700 nm yielded aPAR of 700 Einstein at the face of the fixture. After entering the water from the air and traveling 24 inches through the water this value would be considerably less.
I'm looking around for data on the Finnex Ray 2 at the face of the fixture, if I find something for comparison, I'll post it.
My objective is to build a fixture comparable to the $100 Finnex Ray 2 (24" Model) for half of the material price. (My labor will be free)
My first stop on this journey was to select and test lamps. I found these Chinese waterproof fog lights on ebay. Advertised for vehicle use, I figured they'd be very bright. I got 4 lamp sets delivered to my home for $15.

I know that all lamps are not equal, some enhance plant growth more than others. With some experience in a lab, I wanted to test these lamps to characterize their ability to support photosynthesis. I found this thread on another forum with seemingly reliable info on the Finnex Ray 2. Finnex RAY II & FugeRAY PAR Data
Finnex's LED Spectrum from the thread (And website?)

Measured Spectrum of Chinese Fog Lamp LED Spectrum

So far so good. It looks like I am on the right path. But now I've got to measure the PAR of these Chinese fog lamps for comparison with Finnex's. That's when I opened this thread.
In the lab, I don't have access to a PAR sensitive photometer, and the premise of this thread is I am too cheap to go out and buy one. But I was able to get my hands on a calibrated spectrometer. (This is a VERY expensive device, but I found myself able to borrow one.) My strategy was to use the spectrometer, a few assumptions, and some undergraduate knowledge of physics to crank out a PAR measurement.
First, the assumption:
I assumed that 100% of the power that entered the lamp was converted into light. This isn't true, but truth is not too far off. I measured each lamp dissipated 3.5W, so I assumed that the total amount of light power produced equaled 3.5W.
Then, the spectrometer:
I measured the spectrum of light produced by my new Chinese fog lamps, you can see the graph produced above. The area bound by this curve I assumed to be equal to the 3.5W I measured earlier.
Finally, the physics:
In the early 20th century a few scientists determined that the wavelength a photon was related to the energy of that photon. Having measured the energy delivered by a number of photons with my spectrometer, I divided by the energy per photon to find the number of photons.
Adding all of the photons across all wavelengths of light between 400 and 700 nm yielded aPAR of 700 Einstein at the face of the fixture. After entering the water from the air and traveling 24 inches through the water this value would be considerably less.
I'm looking around for data on the Finnex Ray 2 at the face of the fixture, if I find something for comparison, I'll post it.
Mebbid
Aquarium Advice Addict
You know its a good discussion when people barely understand what's being said. Beyond my caliber of contribution but subscribing none the less.
Brian_Nano12g
Aquarium Advice Addict
This would be a good discussion with Hoppy on TPT. As it stands, it's way over my head 
aqua_chem
Aquarium Advice Addict
.In the lab, I don't have access to a PAR sensitive photometer, and the premise of this thread is I am too cheap to go out and buy one. But I was able to get my hands on a calibrated spectrometer. (This is a VERY expensive device, but I found myself able to borrow one.) My strategy was to use the spectrometer, a few assumptions, and some undergraduate knowledge of physics to crank out a PAR measurement.
Care to share what you're using and how it's calibrated?
In the early 20th century a few scientists determined that the wavelength a photon was related to the energy of that photon. Having measured the energy delivered by a number of photons with my spectrometer, I divided by the energy per photon to find the number of photons.
Adding all of the photons across all wavelengths of light between 400 and 700 nm yielded aPAR of 700 Einstein at the face of the fixture. After entering the water from the air and traveling 24 inches through the water this value would be considerably less.
This is possible, but the calculus involved is pretty involved. Can you post your work for review?
Sent from my iPad using Aquarium Advice
Similar threads
- Replies
- 31
- Views
- 920
- Replies
- 2
- Views
- 133
- Replies
- 2
- Views
- 468
- Replies
- 4
- Views
- 1K
- Replies
- 3
- Views
- 643