jetajockey
come get me tang police!
*WARNING* This guide is intended to give a better understanding of Ammonia toxicity and is not intended to diagnose or treat any problems. Please use this information wisely. *WARNING*
Ammonia. It's bad. I think even the most novice fishkeeper understands this concept. It's a common idea that Ammonia levels should be 0 in a well operating tank, and this is absolutely correct.
Ammonia poisoning is very harsh, and often, when fish begin to show effects of ammonia poisoning, even with swift action to remedy it by water changes and detoxifiers, it may be too late for some.
If you ever get ammonia readings in a cycled tank, the first thing to do is to figure out where they are coming from, and then formulate a plan to remedy it.
In cycling tanks, or even newly established tanks, the ammonia level can rise unexpectedly.
This can also happen if the ph, temperature, or bioload changes. It often takes a dramatic change, but it happens.
Still with me? Time to get a little technical.
Ammonia in aquaria has 2 forms. NH3 (Free Ammonia) and NH+4 (Ammonium).
Ammonium is much less toxic than Free Ammonia is,
so much so that Ammonia detoxifiers such as Prime or Amquel+ rely on converting the Free Ammonia over to Ammonium temporarily.
So to sum it up. Free Ammonia (NH3) + Ammonium (NH+4) = TAN (Total Ammonia Nitrogen). TAN is the total amount of ammonia
found in your tank, in both forms.
While TAN is consistent, the levels of NH3 and NH+4 in your tank fluctuate depending on both temperature and pH levels.
TAN is what your test kit reads in. It does not differentiate between NH3 and NH+4, which means that it gives you the total of both combined.
This is important to know, because one form is far less toxic than the other, and because of that, your tank may be in less/more danger than you think.
And this is why I created charts.
These charts will give you a reference point to see how much of the TAN(total ammonia nitrogen) in your tank is actually
the harmful Free Ammonia (NH3).
These charts were compiled with data taken from Agriculture Water Quality Alliance (AWQA: Agriculture Water Quality Alliance) as well as
the University of Florida's Electronic Data Information Source (EDIS) found at EDIS - Electronic Data Information Source - UF/IFAS Extension
There is a threshold level for Free Ammonia (NH3) in the tank. A threshold level is basically the point at which adverse effects begin to happen. So levels below this are deemed safe.
The threshold level for Free Ammonia (NH3) .025ppm
Levels of .05ppm Free Ammonia (NH3) may harm fish, and at levels of 2.0ppm fish will begin to die.
How to use:
1) Test your water for Ammonia, pH, Temperature.
2) Take your Ammonia reading and find the proper chart
3) Find your pH/Temperature to see where you are on the chart.
The charts are color coded for easy referencing, and read in PPM
GREEN = SAFE YELLOW = CAUTION RED = DANGER
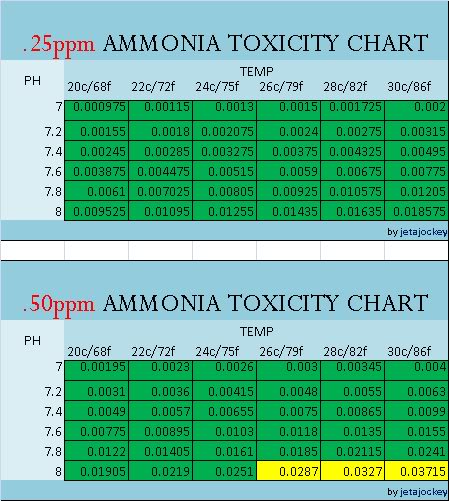
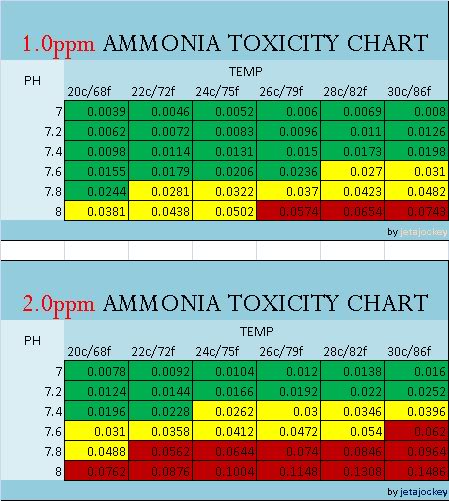
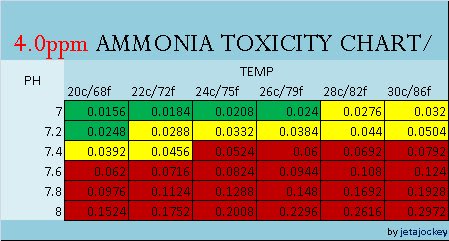
Notes: The charts only go down to 7.0pH. There is no data given for pH levels below this, and I'm not sure why. Rather than guesstimate, I'll leave it as-is for now. There is a common trend that the lower the pH, the less NH3 is present, but do know that there is a point around 6pH where nitrification completely stops.
A lack of buffers in a tank is a problem, especially when cycling, so if you are reading consistently low pH readings (or a wild swing in pH, adding a buffer may be a good idea.
You have your chart reading, what now?
Well, this ultimately comes down to you. If you are in the yellow or red, then a water change definitely should be in short order. This can get complicated if your water source has ammonia in it, so it's always a good idea to test your tap water for ammonia to make sure you aren't fighting an uphill battle.
As always, I recommend a good water conditioner that temporarily detoxifies ammonia to go along with as many water changes as needed to put you back into the green.
I hope this gives you a better understanding of how Ammonia toxicity works and helps you in your cycling process!
Ammonia. It's bad. I think even the most novice fishkeeper understands this concept. It's a common idea that Ammonia levels should be 0 in a well operating tank, and this is absolutely correct.
Ammonia poisoning is very harsh, and often, when fish begin to show effects of ammonia poisoning, even with swift action to remedy it by water changes and detoxifiers, it may be too late for some.
If you ever get ammonia readings in a cycled tank, the first thing to do is to figure out where they are coming from, and then formulate a plan to remedy it.
In cycling tanks, or even newly established tanks, the ammonia level can rise unexpectedly.
This can also happen if the ph, temperature, or bioload changes. It often takes a dramatic change, but it happens.
Still with me? Time to get a little technical.
Ammonia in aquaria has 2 forms. NH3 (Free Ammonia) and NH+4 (Ammonium).
Ammonium is much less toxic than Free Ammonia is,
so much so that Ammonia detoxifiers such as Prime or Amquel+ rely on converting the Free Ammonia over to Ammonium temporarily.
So to sum it up. Free Ammonia (NH3) + Ammonium (NH+4) = TAN (Total Ammonia Nitrogen). TAN is the total amount of ammonia
found in your tank, in both forms.
While TAN is consistent, the levels of NH3 and NH+4 in your tank fluctuate depending on both temperature and pH levels.
TAN is what your test kit reads in. It does not differentiate between NH3 and NH+4, which means that it gives you the total of both combined.
This is important to know, because one form is far less toxic than the other, and because of that, your tank may be in less/more danger than you think.
And this is why I created charts.
These charts will give you a reference point to see how much of the TAN(total ammonia nitrogen) in your tank is actually
the harmful Free Ammonia (NH3).
These charts were compiled with data taken from Agriculture Water Quality Alliance (AWQA: Agriculture Water Quality Alliance) as well as
the University of Florida's Electronic Data Information Source (EDIS) found at EDIS - Electronic Data Information Source - UF/IFAS Extension
There is a threshold level for Free Ammonia (NH3) in the tank. A threshold level is basically the point at which adverse effects begin to happen. So levels below this are deemed safe.
The threshold level for Free Ammonia (NH3) .025ppm
Levels of .05ppm Free Ammonia (NH3) may harm fish, and at levels of 2.0ppm fish will begin to die.
How to use:
1) Test your water for Ammonia, pH, Temperature.
2) Take your Ammonia reading and find the proper chart
3) Find your pH/Temperature to see where you are on the chart.
The charts are color coded for easy referencing, and read in PPM
GREEN = SAFE YELLOW = CAUTION RED = DANGER



Notes: The charts only go down to 7.0pH. There is no data given for pH levels below this, and I'm not sure why. Rather than guesstimate, I'll leave it as-is for now. There is a common trend that the lower the pH, the less NH3 is present, but do know that there is a point around 6pH where nitrification completely stops.
A lack of buffers in a tank is a problem, especially when cycling, so if you are reading consistently low pH readings (or a wild swing in pH, adding a buffer may be a good idea.
You have your chart reading, what now?
Well, this ultimately comes down to you. If you are in the yellow or red, then a water change definitely should be in short order. This can get complicated if your water source has ammonia in it, so it's always a good idea to test your tap water for ammonia to make sure you aren't fighting an uphill battle.
As always, I recommend a good water conditioner that temporarily detoxifies ammonia to go along with as many water changes as needed to put you back into the green.
I hope this gives you a better understanding of how Ammonia toxicity works and helps you in your cycling process!
Last edited by a moderator:

