threnjen
Aquarium Advice Addict
Well 2 mols of acidity are produced for every 1 mole of ammonia oxidized. I do not know how to convert that into usable information though. That's just the chem reaction of oxidation.
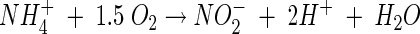
Except that you can have pH higher than "stall level" and be out of kH, can't you?
You can have pH 7.2 water that has 0 kH, or is that incorrect?
Well 2 mols of acidity are produced for every 1 mole of ammonia oxidized. I do not know how to convert that into usable information though. That's just the chem reaction of oxidation.
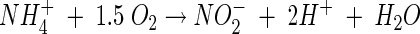
Yes I see what you are saying now. It does make sense.
Look at your posts on the first page when ph starts to drop significantly. Why has kh not budged before ph starts to drop?
No clue, what is in it? I think that "pH down" is phosphoric acid (which would solve at least one person's cycling problems that I can think of in newbie area) but I don't know what pH up is. Probably because there are so many other easy ways to raise pH.How does ph up work. If it doesn't add carbonates then maybe you could use it as an experiment?
Or better still don't add any baking soda at the start of the next cycle. Since your tap water has very low dkh and an averagish ph you could does your 16ppm ammonia and just leave it be. IF it stalls and dkh depletes down to 0 and you add baking soda and it Kick starts again then you have nailed it and you can have the Nobel prize.
The penny has dropped now I do see what you are saying.
But it's not true 8.4?Btw apparently sodium bicarbonate will try to keep the ph level at 8.4 (we know this already) this is where it gets tricky.
If the ph is say 9.4 then the bicarbonates will lend hydrogen ions back to the water to reduce ph to whatever the buffers preferable ph level is. From a baking soda point if view this is 8.4.
But it's not true 8.4?
I read that you can't "overdose" it since it will stop at 8.4. Does the problem only apply if your starting pH is higher than 8.4?
I mean I guess in good news, that is a really rare pH to be so high
Anyone else have bandwidth/supplies to try a "Set it and forget it"? Even if I can do it a dozen times, it's only applicable to my own water. You don't have to test like a spaz like I do, just see if it works. Can do it in a bucket!
You need: filter, heater, ammonia, baking soda, fish flakes (or if you use dry ferts, your phosphate mix)
Yeah I could do that. It might be too expensive though to be worth it (even if we split the shipping). I wonder what the smallest amount I could send would be to keep the weight down. I'll think it over tomorrow what kind of container I could put it in.I do have a 6 gallon I could cycle. You might have to send me some ace ammonia or whatever it's called and I'll give you the money?
"Below 8.4, Bicarbonate absorbs H+ ions and the equation moves to the left, making the water more alkaline. This continues until the pH reaches 8.4 when the process stops"
"Above 8.4, Bicarbonate releases H+ ions and the equation moves to the right, making the water more acidic. This continues until the pH is 8.4 when the process stops."
I forgot to add this bit on to that post before
