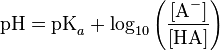Keep in mind, my comment was in relation to fish needing KH to be healthy, not the impact on pH which is in a different spot.While this is true, it translates very poorly into practice, as large bodies of water effectively dilute the acidity, negative acid accumulation somewhat. But in small tanks, the lack of KH is potentially dangerous as there is nothing to prevent pH drops.
Honestly, I think to make this information consumable you should insert a picture here to represent this.About the 10x pH issue: I think your diagram is inconsistent. The way you represented it is that 7 is the middle. That is true if you are referring to BOTH alkalinity AND acidity. As the acidity increases, the alkalinity decreases, so that when the pH=7, the alkalinity and acidity are equal. Basically, with your scale, with numbers under 7 you are referring to the acidity and above 7 you are referring to the alkalinity. Does that make sense? The way your diagram showed it, 7 was the middle. I think you were trying to show both the acidity and the alkalinity but that won't work with the type of diagram you made.
For example: this is a diagram of the acidity and ONLY the acidity changes as the pH changes.
(1)10000000x-(2)1000000x-(3)100000x-(4)10000x-(5)1000x-(6)100x-(7)10x-(8)1x-(9)0.1x-(10)
Something like this:

or this:

Depending on what you are trying to show.