JackBlasto
Aquarium Advice Freak
I know there are a lot as I've found some through google but this is what I'm trying to find...
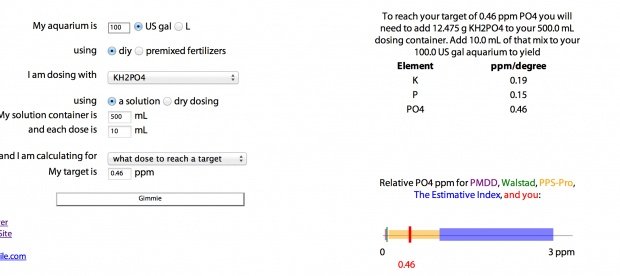
I have dry ferts I'm mixing in 500 ml bottles. The amount of monopotassium phosphate I'm mixing in a pps pro mix is 3 grams for 100 gallons I'm dosing 10 mL of my pps pro solution. Now, testing my phosphates it comes up lacking daily with a result of 0.56. My target phosphate is 1.0 so I make up the difference by adding enough phosphate with another liquid seachem product to compensate. I don't want to do this forever. I want to figure out the ratio of how many grams will up my mix another 0.46 so I can eliminate the seachem liquid stuff from my daily routine.
A lot of the calculators I see ask for the amount I'm dosing in ppm... Well, I don't know what I'm dosing in ppm... I want to achieve a 1ppm reading of phosphate... I know what I'm mixing in grams and I know I'm putting it in a 500 mL bottle along with NO3, etc.
Any help would be MUCH appreciated...
Long question made short:
How many grams of KH2PO4 to increase my phosphates by 0.46 if I'm mixing it in 500 mL bottle and dosing 10 mL daily in a 100 gallon tank?

(hehe... looking over this question I can very roughly just double my 3 to 6 grams and probably achieve my target BUT I'd kinda like to see a calculator or know how I can figure it out so that I can adjust things that aren't in nice half way points in the future etc. In the past I have just dosed and tweaked it based on tedious trial and error and I'd love to eliminate some guesswork in the future saving me a LOT of time by understanding the relationship of grams added to ppm changed.)
I have dry ferts I'm mixing in 500 ml bottles. The amount of monopotassium phosphate I'm mixing in a pps pro mix is 3 grams for 100 gallons I'm dosing 10 mL of my pps pro solution. Now, testing my phosphates it comes up lacking daily with a result of 0.56. My target phosphate is 1.0 so I make up the difference by adding enough phosphate with another liquid seachem product to compensate. I don't want to do this forever. I want to figure out the ratio of how many grams will up my mix another 0.46 so I can eliminate the seachem liquid stuff from my daily routine.
A lot of the calculators I see ask for the amount I'm dosing in ppm... Well, I don't know what I'm dosing in ppm... I want to achieve a 1ppm reading of phosphate... I know what I'm mixing in grams and I know I'm putting it in a 500 mL bottle along with NO3, etc.
Any help would be MUCH appreciated...
Long question made short:
How many grams of KH2PO4 to increase my phosphates by 0.46 if I'm mixing it in 500 mL bottle and dosing 10 mL daily in a 100 gallon tank?
(hehe... looking over this question I can very roughly just double my 3 to 6 grams and probably achieve my target BUT I'd kinda like to see a calculator or know how I can figure it out so that I can adjust things that aren't in nice half way points in the future etc. In the past I have just dosed and tweaked it based on tedious trial and error and I'd love to eliminate some guesswork in the future saving me a LOT of time by understanding the relationship of grams added to ppm changed.)